Which of the Following Sn2 Reactions Is the Fastest
January 11 2022 thanh Rank the SN2 reaction rates for the following compounds. Which of the following alkyl halides reacts the fastest in an SN2 reaction.

Which Of The Following Sn 2 Reactions Is The Fastest
The rate of SN2 reaction in alkyl halide takes place in the order.
. Despite this 3 alkyl halides do undergo nucleophilic substitution reactions quite rapidly but by a different. Velocity of the reaction depends on the concentration of the substrate as well as the nucleophile. S N 2 reactions involve the formation of intermediate transition state thus less hindered alkyl halide readily undergoes S N 2 reaction.
Check out a sample QA here. 2 reactions involve the formation of intermediate transition state thus less hindered alkyl halide readily undergoes S N. Rank the sn2 reaction rates for the following compounds.
The specific rotation of pure R-sec-butyl alcohol is -1352. This answer is not useful. We review their content and use your feedback to keep the quality high.
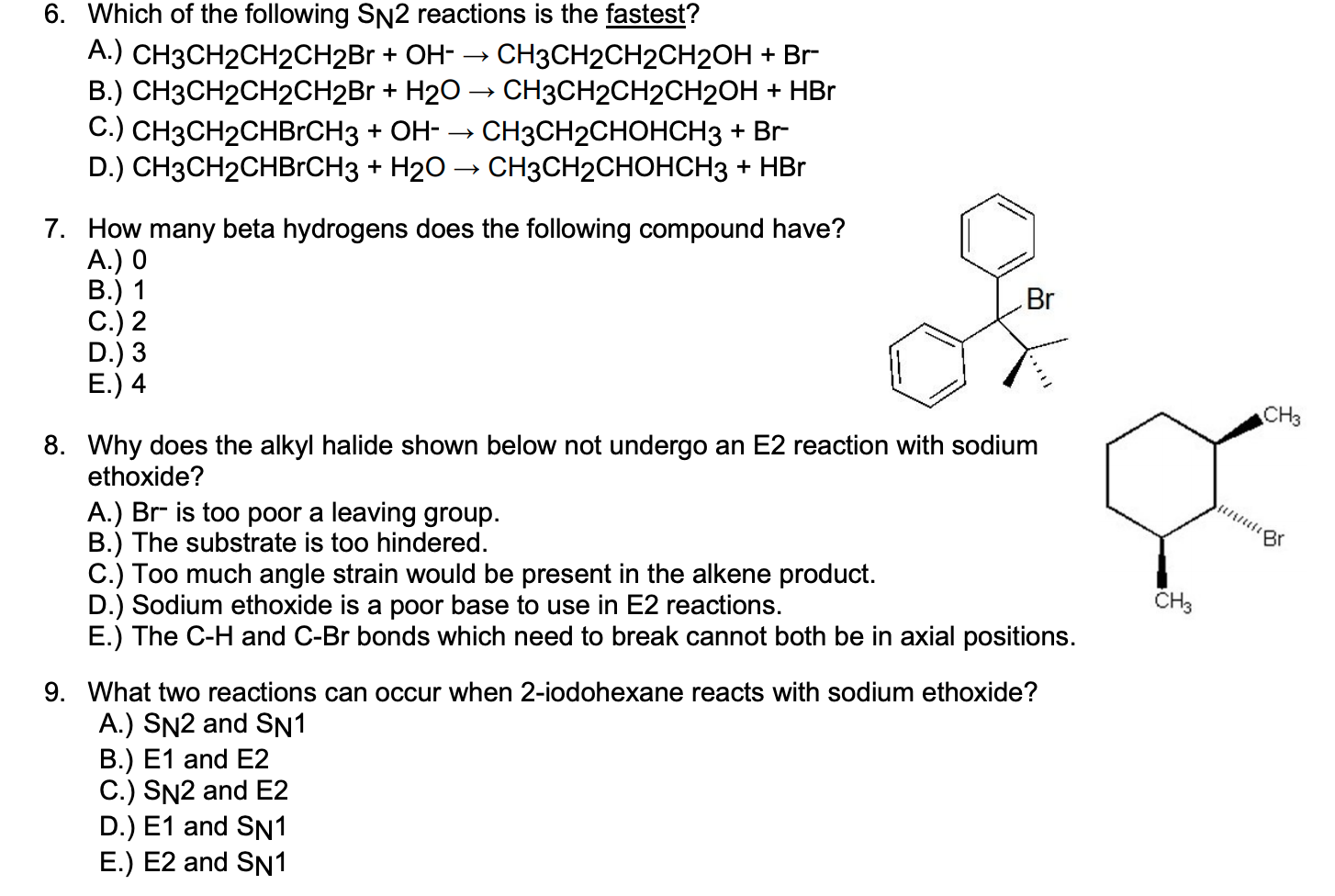
What product is formed when R-2-chloropentane reacts with hydroxide ion in an SN2 reaction. Which of the following SN2 reactions is the slowest. One in which the nucleophilic attack and the.
This answer is useful. Show activity on this post. Yes steric hindrance greatly affects the rate of reaction because steric hindrance increases the activation energy of reaction decreasing the rate of reaction.
Experts are tested by Chegg as specialists in their subject area. Fastest rate 3-chloro-3-methylpentane 2-chloro-3-methylpentane 1-chloro-3-methylpentane chloromethane slowest rate. CH3CH2CH2CH2Br OH CH3CH2CH2CH2OH Br- - O A.
Moreover C I bond is less stable than C B r or C C l bond thus alkyl iodides are more reactive as compared to other halides. Option e C H 3 2 C H C l. The term SN2 stands for Substitution Nucleophilic Bimolecular.
A 2-chloro-2-methylpropane B 2-chlorobutane C 1-chlorobutane D chloromethane. Two reacting species are involved in the rate determining step of the reaction. The reaction is favoured by strong N u and in the presence of polar aprotic solvent optically active halides give Walden inversion by S N 2 mechanism.
View the full answer. Want to see the full answer. This compound is a secondary alkyl halide.
During the transition state the bond to the nucleophile forms at the same time that the bond to the leaving group breaks. Polar aprotic solvent is the best solvent for S N 2 reaction as it only solvates cations and it does not alter the reactivity of nucleophiles. The one with the minimum hindrance will be the most likely to undergo SN2 reaction.
The S N 2 reaction is a nucleophilic substitution reaction where a bond is broken and another is formed synchronously. As a reminder from the introduction to nucleophilic substitutions these are reactions where the nucleophile replaces the leaving group. NaSCH3 CH3-CH2-CH2-Br DMSO NaOCH3 CH3-CH2-CH2-Br H20 NaOCH3 CH3-CH2-CH2-Br DMSO NaSCH3 CH3-CH2-CH2-Br H20 Expert Solution.
This type of reaction is also referred to as bimolecular nucleophilic. C H X 3 C H B r will give faster S N 2 reaction because when a nucleophile will approach C H X 2 C H B r for S N 2 reaction the double bond between C H X 2 C H will hinder its approach steric effect but there is no such hindrance in case of C H X 3 C H X 2 B r. S N 2 reaction is bimolecular reaction which takes place by the formation of TS.
Moreover C I bond is less stable than C Br or C-Cl bond thus alkyl iodides are more reactive as compared to other halides. Want to see the full answer. 1 Which of the following alkyl halides reacts the fastest in an SN2 reaction.
Which of the following SN2 reactions is the fastest. Is S N 2 rate governed by steric effects. Hence C H 3.
Which of the following SN2 reactions proceeds the fastest. In this post we will talk about the S N 2 mechanism of nucleophilic substitution reactions. The presence of hetro group atom at β C atom in.
Hence C H 3 C H 2 I is most reactive towards S N 2 reaction. View the full answer. CH3CH2CH2CH2Br H2O CH3CH2CH2CH2OH HBr CH3CH2CHBICH3 OH- - CH3CH2CHOHCH3 Br- OC.
Thus the nucleophile needs to approach from the back and configuration. These reactions are divided in two main types. Rank the SN2 reaction rate of the following species from fastest to slowest.
Br is a better leaving group than F as C-F bond is huge strong du. Which of the following SN2 reactions is the fastest. Which of the following does not provide evidence that there are two different mechanisms for nucleophilic substitution.
ANS- B REACTION will be the fastest SN2 reaction b. 37 1st Order Nucleophilic Substitution Reactions ie S N 1 reactions C CH 3 H 3 C CH 3 Br Na I-C CH 3 H 3 C CH 3 I Na Br-3 rapid 3 alkyl halides are essentially inert to substitution by the S N 2 mechanism because of steric hindrance at the back side of the a-carbon. A quick SN2 reminder.
Primary secondary tertiary. Now let us look at the substrates. The SN2 reaction is a single-step displacement of a leaving group by a nucleophile.
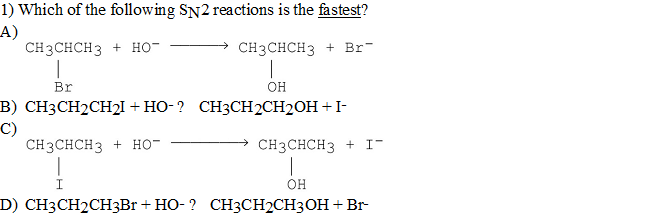
Solved Which Of The Following Sn2 Reactions Is The Fastest Chegg Com
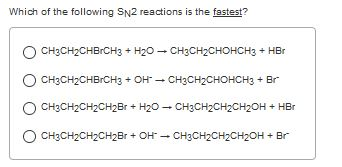
Solved Which Of The Following Sn2 Reactions Is The Fastest Chegg Com

Solved 6 Which Of The Following Sn2 Reactions Is The Chegg Com
No comments for "Which of the Following Sn2 Reactions Is the Fastest"
Post a Comment